|Intro| - |Sizes| - |Tour| - |Help|
[Quarks] - [Nucleons] - [Nucleus] - <Atom> - [Cell] - [People]
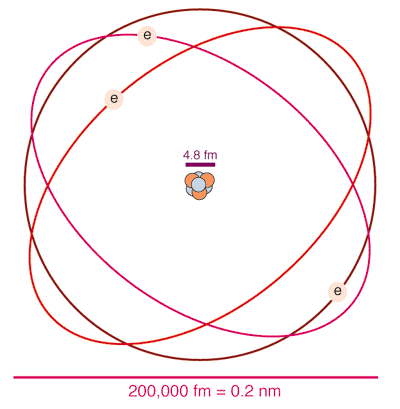
An atom is roughly about 0.2 nm across (nm means nano meter, where nano is the metric prefix meaning 10-9, sometimes written 10^{-9} when superscripts are not available in text), although the electrons spend most of their time in a region about 0.1 nm = 0.0000000001 meters in diameter.
The nucleus is much smaller than an atom. A light nucleus like our Lithium example is only about 0.000005 nm across. One way of understanding the scale factor of about 20,000 to 40,000 between the size of an atom and the size of its nucleus is to compare an atom to a large stadium like the Rose Bowl. If the electron "orbits" out around the outer edge of the stadium, the nucleus would be the size of a dime or quarter sitting at midfield.
Lithium has 3 protons, either 3 or 4 neutrons, and 3 electrons. The chemistry of this element is determined by the +3 charge on its nucleus, the number of protons, not the number of neutrons.

The size of the nucleus in this cartoon has been exagerated to allow us to see the protons and neutrons.
The sketch above shows the electrons in "orbits" like those used in the old Bohr model of the atom. This model makes it easy to draw pictures of an atom like a small solar system, but it is not accurate. The electrons in an atom follow the rules of quantum mechanics , which means they do not follow a specific path. The real situation is better represented by the following picture:

In reality, the electron position is smeared out over a fuzzy region sort of like we show above. They can be found quite close to the nucleus or quite far away, but on average they are found within a region about 0.1 to 0.3 nm across. We quote that average as the "size" of an atom up above. Atoms in a solid are typically about 0.2 to 0.3 nm apart, with the outer electron "orbitals" essentially touching as they interact to form the bonds between the atoms.